Sample Preparation
Below you will find guidelines and recommendations concerning sample preparation. Please contact the laboratory if you have supplementary questions.

- Please fill the tube correctly to avoid artifacts due to low sample volume. Be sure to mix the blood adequately with the anticoagulant by gentle inversion.
- Make a fresh blood smear at the time of blood collection and enclose the unstained preparation with the sample tubes. This will ensure an optimal morphological evaluation.
- Test results will be most valid if samples are less than 24 hours old when arriving at the laboratory.
- Serum tubes should be left horizontally for 15-20 minutes at room temperature after collection.
- Centrifugation.
- Remove the serum and place in an Eppendorf tube or other suitable container.
- The patient should be fasting before collection to avoid postprandial lipemia.
- When collecting samples on Fridays we recommend storing samples in a refrigerator and shipping Sunday/Monday.

Please contact the hospital for specific recommendations concerning additional parameters.
- Signalement: Patient species, breed, age and sex.
- Localization and thorough description of the tumor/effusion.
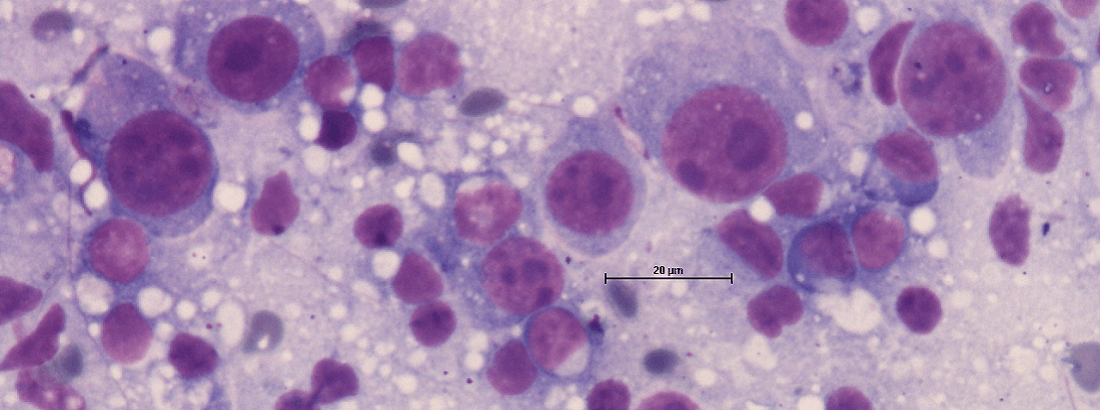
- Other relevant information, e.g. information regarding diagnostic findings by x-ray, ultrasound, CT/MRI etc. When submitting samples from liver, spleen and/or bone marrow it is furthermore recommended to enclose a CBC printout and/or a fresh blood smear.
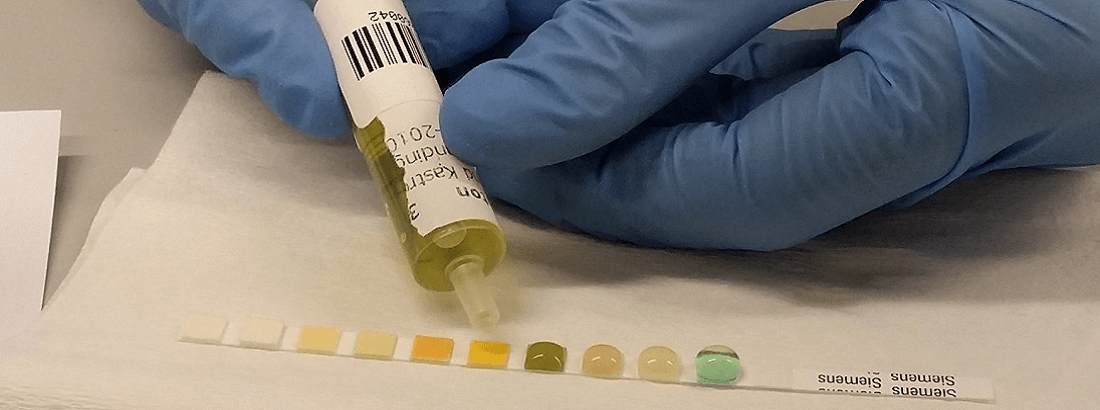
- To examine urine sediment, please submit an unstained, air-dried line smear. For guidance please look through our videomaterial in the menu to the right.
- Remaining urine should be submitted in a suitable close-fitting container.
- The sample should be refrigerated until submission.
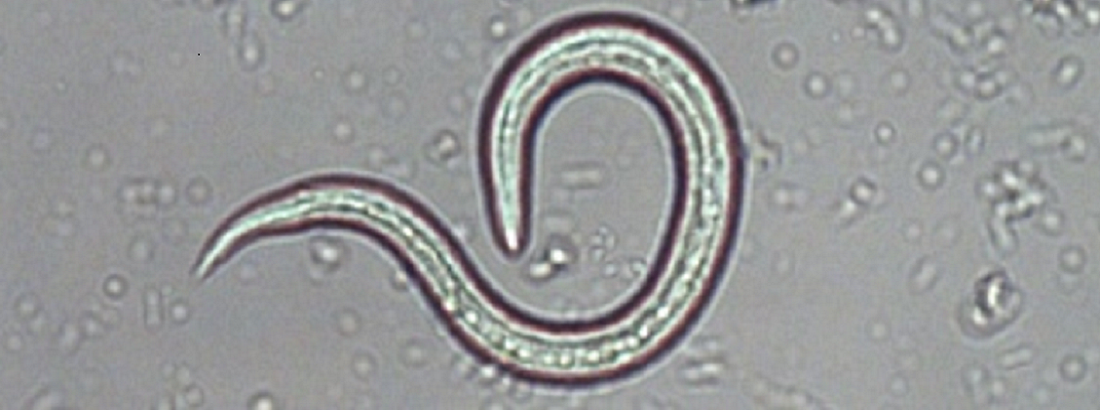
- Feces is collected over three consecutive days.
- The feces must not have been in direct contact with the ground.
- We recommend using a plastic bag for collection.
- Please fill up the sample tube.
- The samples should be refrigerated until submission.
- Use a citrate tube (3,2% citrate) for coagulation assays.
- It is essential to ensure a clean venipuncture (atraumatic) and a rapid blood flow when collecting blood for coagulation assays.
- Use a "waste" citrate-tube before filing the sample tube, so to prevent coagulation by contamination with tissue factor.
- The sample tube should be filled correctly to ensure the appropriate ratio between anticoagulant and blood.
- Gently invert the tube 8-10 times to mix the blood with the anticoagulant.
- Centrifugation.
- Plasma is removed to an Eppendorf tube or other suitable container.
- To be analysed within 3 hours of sample collection or
- The sample should be frozen ( minus 80 degrees celcius) and kept frozen until it reaches the laboratory by prior agreement.
Guidelines and videomateriale
Sample volume
Case: Incorrect sample volume (in danish)
Blood smear
Blood smear (in danish)
Cytologi
Urine analysis
